-
- Chemical Hygiene & Laboratory Safety Manual
- Laboratory Emergency Guidance
- Chemical Use in CARE
- Personal Protective Equipment
- Chemical Fume Hoods
- Chemical Storage & Compatibility
- Hydrofluoric Acid Safety Awareness
- Compressed Gas Cylinder Safety
- Nanomaterials Safety
- Heating Element Safety
- Methylene Chloride Exposure Control Program
Environmental Health and Safety
Hydrofluoric Acid Safety Awareness
Sub Navigation
- Chemical Hygiene & Laboratory Safety Manual
- Laboratory Emergency Guidance
- Chemical Use in CARE
- Personal Protective Equipment
- Chemical Fume Hoods
- Chemical Storage & Compatibility
- Hydrofluoric Acid Safety Awareness
- Compressed Gas Cylinder Safety
- Nanomaterials Safety
- Heating Element Safety
- Methylene Chloride Exposure Control Program
Effects of Hydrofluoric Acid
Hydrofluoric acid (HF) can burn through glass or concrete. Fluoride ions are absorbed through the skin, destroying tissue until they are sequestered in the bones. HF damage causes severe, long-term pain and slow-healing burns and can be deadly. Significant delays between exposure and symptom onset may occur.
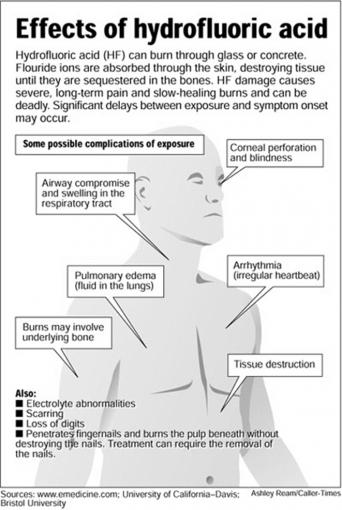
Some possible complications of exposure
- Airway compromise and swelling in the respiratory tract
- Corneal perforation and blindness
- Pulmonary edema (fluid in the lungs)
- Arrhythmia (irregular heartbeat)
- Burns may involve underlying bone
- Tissue destruction
- Electrolyte abnormalities
- Scarring
- Loss of digits
- Penetrates fingernails and burns the pulp beneath without destroying nails. Treatment can require the removal of the nail